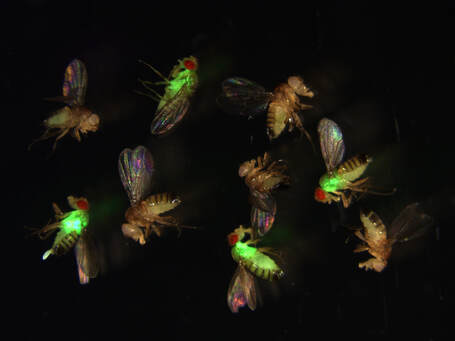
 Drosophila infected by green fluorescent bacteria.
Red-eyed flies lacking antimicrobial peptides are
susceptible to infection, while white-eyed flies with
antimicrobial peptides resist infection.
Drosophila infected by green fluorescent bacteria.
Red-eyed flies lacking antimicrobial peptides are
susceptible to infection, while white-eyed flies with
antimicrobial peptides resist infection.
At the interface of host-pathogen interactions
I study the evolution of the immune system. Because surviving infections is so important, our immune system is adapted to fight many parasites and pathogens. My work has focused on immune effectors: i.e. the things that directly kills germs. I've found that these effectors aren't always just "kill-everything" genes, but some of them are actually targeted silver bullets that fight specific germs we are commonly exposed to. It's a profound realisation that our genes, our genomes, are shaped so strongly by specific external microbes.
Taking a step back: evolution of immune signalling
In my ongoing work, I am taking a step back to ask how these genes are regulated across species. A basic assumption we all make about how immunity "works" is that specific genes do specific things - and to be clear, that's often a useful way to think about it!
We then make these models. We assemble "pathways" or "cascades" of genes/proteins activating each other one after another. This is the sort of process, immune pathway activation, that leads to inflammation. We also know that some types of immune pathways are found in all animals. For instance, "Toll-Like Receptors" or "TLRs" were first described as immune genes in fruit flies, but later we realized they are also super important in immunity across the tree of life, including humans. But we also know that these processes work differently in different animals. For instance, in humans, TLRs sense things on the surface of bacteria, but in fruit flies, something else senses the bacteria, and transmits that information to TLRs. So we know they're important in immunity across the tree of life, but we also know that they don't always work the same way.
My lab at the University of Exeter Penryn will be investigating the rules behind this similarity/difference. Using many Drosophila species that are as genetically diverse as humans are from kangaroos, I will ask just how different immune signalling can get within a group of animals. Does the immune system of all fruit flies look like the classic model organism Drosophila melanogaster? Or do some fruit flies have immune systems that work very differently? My lab's goal is to uncover those differences where they do exist, and understand what makes them different. Understanding those principles of immune evolution will help us understand the fundamental forces that make related species respond differently to the same infections.
I study the evolution of the immune system. Because surviving infections is so important, our immune system is adapted to fight many parasites and pathogens. My work has focused on immune effectors: i.e. the things that directly kills germs. I've found that these effectors aren't always just "kill-everything" genes, but some of them are actually targeted silver bullets that fight specific germs we are commonly exposed to. It's a profound realisation that our genes, our genomes, are shaped so strongly by specific external microbes.
Taking a step back: evolution of immune signalling
In my ongoing work, I am taking a step back to ask how these genes are regulated across species. A basic assumption we all make about how immunity "works" is that specific genes do specific things - and to be clear, that's often a useful way to think about it!
We then make these models. We assemble "pathways" or "cascades" of genes/proteins activating each other one after another. This is the sort of process, immune pathway activation, that leads to inflammation. We also know that some types of immune pathways are found in all animals. For instance, "Toll-Like Receptors" or "TLRs" were first described as immune genes in fruit flies, but later we realized they are also super important in immunity across the tree of life, including humans. But we also know that these processes work differently in different animals. For instance, in humans, TLRs sense things on the surface of bacteria, but in fruit flies, something else senses the bacteria, and transmits that information to TLRs. So we know they're important in immunity across the tree of life, but we also know that they don't always work the same way.
My lab at the University of Exeter Penryn will be investigating the rules behind this similarity/difference. Using many Drosophila species that are as genetically diverse as humans are from kangaroos, I will ask just how different immune signalling can get within a group of animals. Does the immune system of all fruit flies look like the classic model organism Drosophila melanogaster? Or do some fruit flies have immune systems that work very differently? My lab's goal is to uncover those differences where they do exist, and understand what makes them different. Understanding those principles of immune evolution will help us understand the fundamental forces that make related species respond differently to the same infections.
 "Time flies like an arrow, fruit flies like a banana." My research has shown that not only do flies like a banana, but the banana presents a niche filled with certain bacteria, and those bacteria impose selection on the fly immune system in highly specific ways. This pattern holds for flies with different ecologies, like mushroom-feeders or plant parasites.
Article synopsis here: https://www.science.org/doi/10.1126/science.adg5725
Full text (free) here: https://mahansonresearch.weebly.com/uploads/1/2/3/6/123603726/hanson_science.adg5725.pdf
Art by https://sci-flies.com/
"Time flies like an arrow, fruit flies like a banana." My research has shown that not only do flies like a banana, but the banana presents a niche filled with certain bacteria, and those bacteria impose selection on the fly immune system in highly specific ways. This pattern holds for flies with different ecologies, like mushroom-feeders or plant parasites.
Article synopsis here: https://www.science.org/doi/10.1126/science.adg5725
Full text (free) here: https://mahansonresearch.weebly.com/uploads/1/2/3/6/123603726/hanson_science.adg5725.pdf
Art by https://sci-flies.com/
Why fruit flies?
Drosophila fruit flies are a great model for my work since they are easy to rear, grow quick, are cheap to care for, and have the same sorts of immune response genes as humans (e.g. "Toll-like receptors"). It also helps that I don't feel personally too too bad about stabbing a fruit fly with a bacteria-laced needle. I'd have to juggle far more ethical implications to carry out my work on another animal model like lab mice. But flies? Eh, whatever. Not only that, the Drosophila immune response is both simpler than humans or mice, and incredibly well-studied. That means it's easy to observe something new and then understand what it means in a broader context. But I should say that fly research also directly translates to other animals, including economically-important insects like the terrifying, jigsaw-wielding Spotted-wing Drosophila "Drosophila suzukii" (OoooOooh...), sandflies, mosquitoes, insects, and other invertebrates (shellfish, crustaceans).
Drosophila fruit flies are a great model for my work since they are easy to rear, grow quick, are cheap to care for, and have the same sorts of immune response genes as humans (e.g. "Toll-like receptors"). It also helps that I don't feel personally too too bad about stabbing a fruit fly with a bacteria-laced needle. I'd have to juggle far more ethical implications to carry out my work on another animal model like lab mice. But flies? Eh, whatever. Not only that, the Drosophila immune response is both simpler than humans or mice, and incredibly well-studied. That means it's easy to observe something new and then understand what it means in a broader context. But I should say that fly research also directly translates to other animals, including economically-important insects like the terrifying, jigsaw-wielding Spotted-wing Drosophila "Drosophila suzukii" (OoooOooh...), sandflies, mosquitoes, insects, and other invertebrates (shellfish, crustaceans).
I did my PhD in Dr. Bruno Lemaitre's lab at l'École Polytechnique Fédérale de Lausanne (EPFL), in Lausanne Switzerland. After this I spent some time as a post-doc and research associate in the Lemaitre lab and Dr. Ben Longdon's lab at the University of Exeter, Penryn, UK funded by the Swiss National Science Foundation (SNSF). I am currently a Wellcome Trust Early Career fellow working at the University of Exeter Penryn to better understand the evolution of host immune signalling, working in collaboration with Dr. Longdon on understanding the factors leading to different infection outcomes across species.
